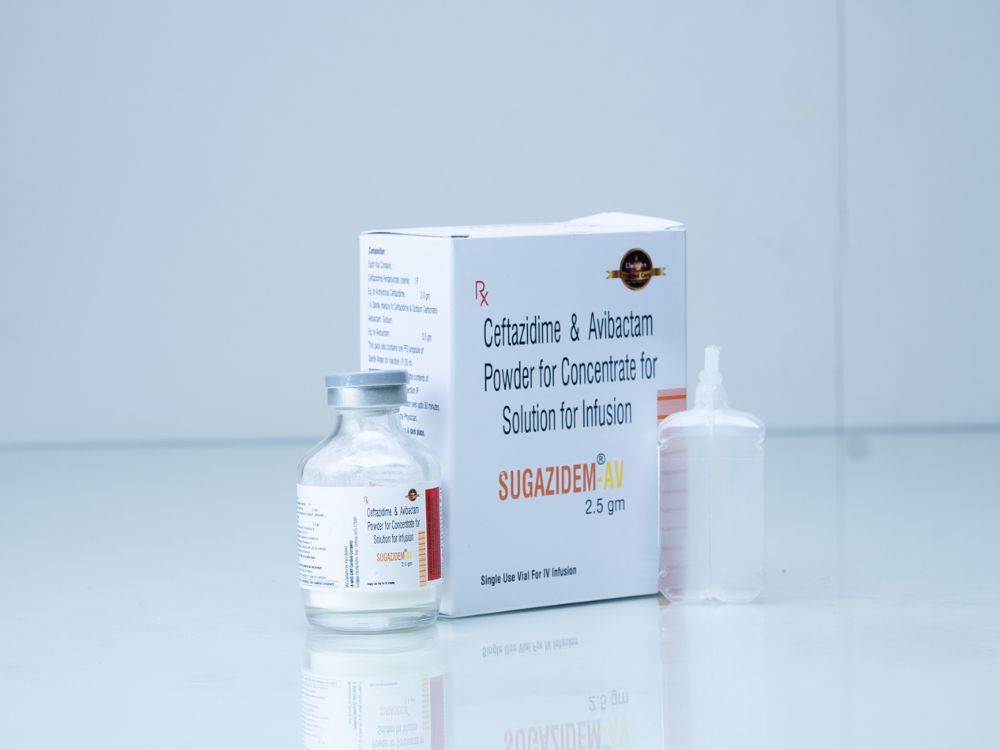
Description
Composition (Per Vial):
-
Ceftazidime – 2000 mg
-
Avibactam Sodium – Equivalent to 500 mg Avibactam
Form: Powder for reconstitution
Route of Administration: Intravenous infusion only
Pack Size: Single-use vial with sterile diluent
Category: Anti-MDR Antibiotic | β-lactam/β-lactamase inhibitor | ICU/critical care therapy
Product Description:
SUGAZIDEM® – AV combines Ceftazidime, a third-generation cephalosporin, with Avibactam, a novel non-β-lactam β-lactamase inhibitor. This cutting-edge combination provides extended-spectrum activity against serious multi-drug resistant Gram-negative bacteria, including Klebsiella pneumoniae carbapenemase (KPC) and OXA-48 producing Enterobacteriaceae.
Used in life-threatening infections where other antibiotics have failed, SUGAZIDEM® – AV is a frontline choice in hospital-acquired infections (HAIs), complicated UTIs, RTIs, and intra-abdominal infections.
Key Benefits:
-
Active against carbapenem-resistant Enterobacteriaceae (CRE) and Pseudomonas aeruginosa
-
Excellent option for ICU patients with limited treatment choices
-
High penetration in lungs, urinary tract, and intra-abdominal tissues
-
Preserves carbapenems as a last-resort option
How It Works:
-
Ceftazidime inhibits bacterial cell wall synthesis, causing lysis.
-
Avibactam protects ceftazidime from degradation by Class A, C, and some D β-lactamases (including KPC & OXA-48), restoring its antibacterial effect.
This synergistic mechanism overcomes key resistance mechanisms seen in ESBLs, AmpC, and carbapenemase-producing organisms.
How to Use:
-
Reconstitute with the provided diluent and further dilute in 100ml NS
-
Administer as IV infusion over 2 hours
-
Recommended dose: 2.5g (Ceftazidime 2g + Avibactam 0.5g) every 8 hours
-
Dose modification needed in renal impairment
-
For hospital and ICU use only under specialist supervision
Safety Advice:
-
Monitor renal function and adjust dose accordingly
-
Watch for Clostridioides difficile-associated diarrhea during/after therapy
-
Avoid in patients with severe hypersensitivity to β-lactams
-
Not recommended in children <18 years unless prescribed by an infectious disease specialist
Precautions:
-
Contraindicated in patients with:
-
Known β-lactam allergy
-
Severe renal dysfunction without dose adjustment
-
-
Caution in:
-
Elderly, immunocompromised, or those with severe hepatic impairment
-
-
Use only when sensitivity confirmed or highly suspected MDR infection
-
Use within 12 hours of reconstitution if stored at 2–8°C
-
Do not mix with other drugs or infuse concurrently in the same line




Reviews
There are no reviews yet.