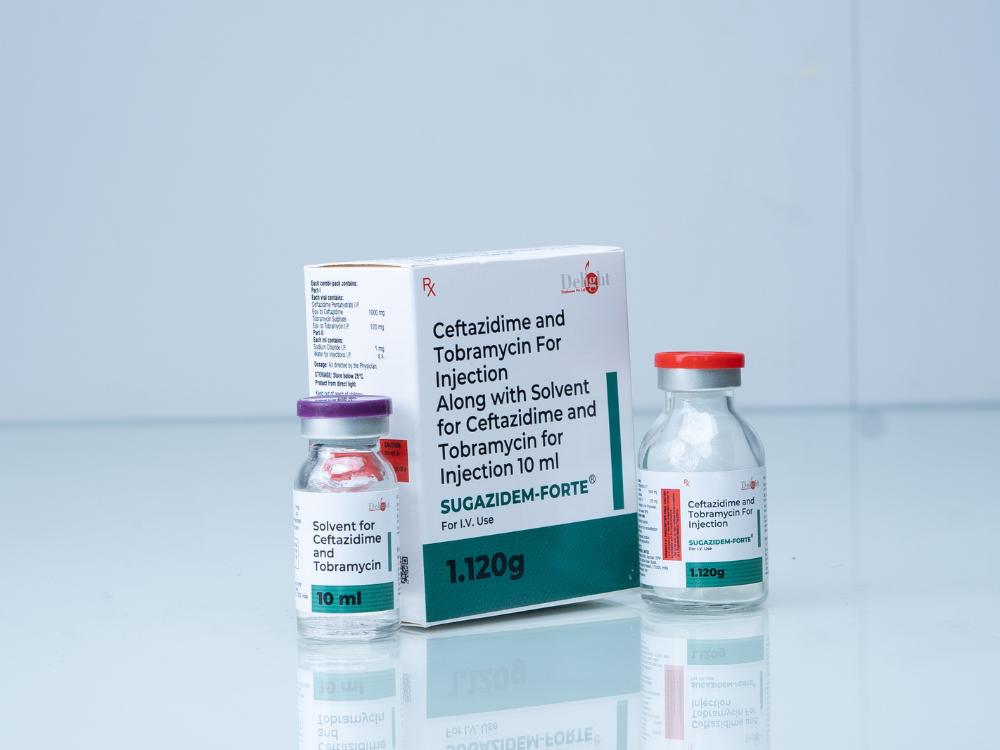
Description
Composition (Per Vial):
-
Ceftazidime – 1000 mg
-
Tobramycin Sulphate – Equivalent to 120 mg Tobramycin base
Form: Dry powder for reconstitution
Route of Administration: Intravenous (IV) or Intramuscular (IM)
Pack Size: Single-use vial with sterile water for injection
Category: Broad-spectrum Antibiotic | Anti-Pseudomonal Injection
Product Description:
SUGAZIDEM® – FORTE is a potent combination of Ceftazidime, a third-generation cephalosporin, and Tobramycin, a powerful aminoglycoside. Together, they deliver synergistic action against a wide range of aerobic Gram-negative organisms, including Pseudomonas aeruginosa, making it ideal for serious hospital-acquired and resistant infections.
This combination is often used in ICU settings, post-surgical infections, and in patients with sepsis or complicated UTIs, RTIs, and peritonitis.
Key Benefits:
-
Potent dual-action targeting multi-drug-resistant Gram-negative bacteria
-
Excellent efficacy in nosocomial infections, including ventilator-associated pneumonia
-
Effective against Pseudomonas, Klebsiella, E. coli, Proteus, and Enterobacter
-
Suitable for use in ICU, critical care, and emergency settings
How It Works:
-
Ceftazidime inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins, causing cell lysis.
-
Tobramycin binds to the 30S ribosomal subunit, inhibiting protein synthesis and causing bacterial death.
The combination provides enhanced bactericidal activity, particularly against difficult-to-treat organisms like Pseudomonas.
How to Use:
-
Reconstitute with the provided sterile water before use
-
Administer via slow IV injection/infusion or deep IM injection
-
Typical adult dose: 1 vial every 8–12 hours, adjusted based on renal function and severity
-
To be used under supervision in a hospital or ICU setting only
Safety Advice:
-
Monitor renal function before and during treatment, especially in elderly or renal-impaired patients
-
Perform audiometry if long-term use is planned, as aminoglycosides may cause ototoxicity
-
Do not mix with other drugs in the same syringe or infusion line
-
Always reconstitute freshly before administration
Precautions:
-
Use with caution in:
-
Patients with existing kidney impairment
-
Concurrent use of other nephrotoxic or ototoxic agents
-
-
Contraindicated in individuals with:
-
Known hypersensitivity to cephalosporins or aminoglycosides
-
-
Prolonged use may lead to secondary fungal or bacterial infections
-
Store in a cool, dry place below 25°C
-
Use immediately after reconstitution – single-use vial only



Reviews
There are no reviews yet.